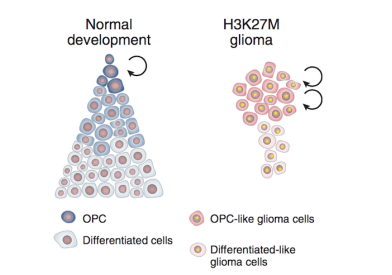
What Is Diffuse Intrinsic Pontine Glioma?
Diffuse intrinsic pontine glioma (DIPG) is a brain tumor that is highly aggressive and difficult to treat. It occurs in an area of the brainstem (the lowest, stem-like part of the brain) called the pons, which controls many of the body’s most vital functions such as breathing, blood pressure, and heart rate. Because of its location in the brain and how rapidly it progresses, DIPG is a “high grade” malignant brain tumor.
- DIPG is a glial tumor, meaning it arises from the brain's glial tissue that supports and protects the brain's neurons.
- It accounts for 10 percent of all childhood central nervous system tumors.
- Approximately 300 children in the U.S. are diagnosed with DIPG each year.
- While DIPG is usually diagnosed when children are between the ages of five and nine, it can occur at any age in childhood.
- It occurs in boys and girls equally and does not generally appear in adults.
At Dana-Farber/Boston Children's Cancer and Blood Disorders Center, we assemble an expert team of pediatric brain tumor specialists with experience treating DIPG to care for your child. They come together in our Childhood Glioma Program, a part of our comprehensive Brain Tumor Center.
Symptoms of Childhood DIPG
The symptoms of DIPG usually develop very rapidly before diagnosis, reflecting the fast growth of these tumors. Most patients start experiencing symptoms very shortly before diagnosis. The most common symptoms include:
- Rapidly developing problems controlling eye movements, facial expressions, speech, chewing, and swallowing (due to problems in the cranial nerves).
- Weakness in the arms and legs.
- Problems with walking and coordination.
How We Diagnose Childhood DIPG
We typically diagnose DIPG through advanced imaging studies. With recent advances in neurosurgical techniques, we can now safely biopsy DIPG. Your child’s doctor will discuss whether a biopsy is an option for your child.
Your child’s medical team will review the diagnostic test results and recommend the best treatment options available to you and your family.
How We Treat Childhood DIPG
DIPG treatment may include:
- Radiation therapy: We typically use radiation as the first line of treatment after diagnosis. It uses high-energy waves from a specialized machine to damage or shrink tumors. Conventional limited-field radiation produces responses in more than 90 percent of children with DIPG. These responses are short-lived, however, lasting about six to nine months on average.
- Experimental chemotherapy: Researchers are actively investigating chemotherapy and biologic therapy as a DIPG treatment. With a biopsy of the tumor at diagnosis, doctors can determine the selection of drugs targeted to your child's tumor. Multiple clinical trials have demonstrated that routine chemotherapy does not increase survival rates for this condition.
Surgery isn’t an option for DIPG because of its location in the brain stem.
We offer extensive support before, during, and after treatment. Once treatment is complete, we continue to care for children and their families through our pediatric cancer survivorship programs, including the Stop & Shop Family Pediatric Neuro-Oncology Outcomes Clinic for pediatric brain tumor survivors. These services address health and social issues, ranging from motor function evaluation and physical therapy to return-to-school and learning programs.