Study suggests possible way to ‘Smac’ cancer
RESEARCH SUMMARY
Study Title: Structures of BIRC6-Client Complexes Provide Mechanism of Smac-Mediated Release of Caspases
Publication:
Science, February 9, 2023, https://doi.org/10.1126/science.ade5750
Dana-Farber Cancer Institute authors:
Eric S. Fischer. PhD, Moritz Hunkeler, PhD, Cyrus Y. Jin
Summary:
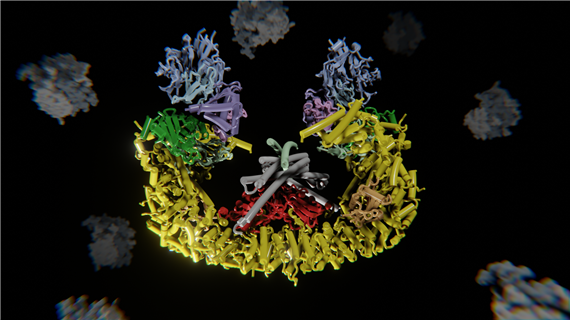
In animals, a process of programmed cell death called apoptosis ensures cells die when they should. An opposing force, governed by inhibitor of apoptosis proteins (IAPs), guards against excessive cell death. Together these competing cellular programs help maintain a balance between unchecked cell growth, such as cancer, and excess cell death, as seen in degenerative disease. In a new study, researchers at Dana-Farber Cancer Institute used cryo-electron microscopy to reveal for the first time how one IAP, a protein called BIRC6, operates at a molecular level to inhibit programmed cell death. They found that BIRC6 proteins pair to form a bowl. At the bowl’s center are receptors that capture caspases, leading to degradation of the caspases and preventing them from triggering cell death. This anti-apoptotic activity can be foiled by a protein called Smac, which also binds to the receptors inside the bowl. When caspases and Smac are both present, a tug-of-war between the forces of cell death and cell preservation ensues. If Smac wins and binds to the BIRC6 complex, caspases are free to trigger apoptosis. Further, Smac binds tightly, irreversibly, and durably to BIRC6, so it effectively shuts down any anti-apoptotic activity of BIRC6.
Impact:
IAPs such as BIRC6 have been attractive targets for cancer drugs because shutting them down in the context of cancer could encourage cancer cell death. Drug developers have created several generations of drugs that mimic the Smac protein, but they have shown limited benefits to patients in clinical trials. This new research opens the door to the design of more effective Smac-mimetic drugs by revealing ways to directly target BIRC6.
Funding:
This research was funded by a National Institutes of Health grant and the Mark Foundation Emerging Leader Award.
Media Contacts
If you are a journalist and have a question about this story, please call 617-632-4090 and ask to speak to a member of the media team, or email media@dfci.harvard.edu.
The Media Team cannot respond to patient inquiries. For more information, please see Contact Us.