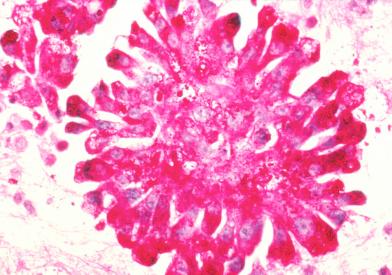
What Is Ewing Sarcoma?
Ewing sarcoma is a type of cancer that forms in bone or soft tissue. It is also called peripheral primitive neuroectodermal tumor (pPNET).
Ewing sarcoma may be found in the bones of the legs, arms, feet, hands, chest, pelvis, spine, or skull. Ewing sarcoma also may be found in the soft tissue of the trunk, arms, legs, head and neck, abdominal cavity, or other areas. Ewing sarcoma is most common in adolescents and young adults.
Why Choose Us
At the Sarcoma Center at Dana-Farber Brigham Cancer Center, our team of experts work together to provide compassionate and highly coordinated care for patients with Ewing Sarcoma. Our center includes leaders in the field who provide our patients with the highest level of care in state-of-the-art facilities. We also perform leading-edge basic scientific and clinical research, with numerous opportunities for patients to participate in clinical trials.
We provide comprehensive services to patients with these cancers, including:
- Access to the highest quality clinical research and new drugs available for the treatment of Ewing Sarcoma
- Support services, including nutrition, complementary therapies, spiritual support, and financial help
- Survivorship programs for patients, plus resources for families and young adults
- Multidisciplinary care delivered by specialists from Dana-Farber Cancer Institute and Brigham and Women’s Hospital
Learn about Ewing sarcoma and find information on how we support and care for people with Ewing sarcoma before, during, and after treatment.
The following information is from the National Cancer Institute